Studies on high-temperature steam and co-electrolysis

Contact: Dr.-Ing. Marina Bockelmann
Within the framework of the energy turnaround, the aim is to completely abandon fossil fuels. In addition to energy sources, however, raw materials are also needed for the chemical industry, which today are also generally obtained from the processing of crude oil or natural gas. One alternative based on renewable energy is the power-to-syngas concept, in which renewable energy is used to convert water and carbon dioxide into hydrogen and carbon monoxide (synthesis gas). A wide variety of hydrocarbons such as methanol, dimethyl ether (DME) or olefins, which are needed in today's chemical industry, can be produced from synthesis gas by downstream synthesis processes. Synthetic fuels such as kerosene can also be produced sustainably from synthesis gas.
A very efficient and ecologically favourable method of synthesis gas production is high-temperature steam and co-electrolysis, if electricity from renewable energy sources is used for this purpose. In high-temperature steam electrolysis (HTEL), water is converted to hydrogen and oxygen at temperatures between 700 °C and 900 °C in a solid oxide electrolyzer cell (SOEC):
H2O → H2 + ½ O2
Subsequently, according to the water-gas equilibrium, the hydrogen obtained can be used to reduce carbon dioxide to carbon monoxide and thus produce synthesis gas:
CO2 + 2 H2 ⇌ CO + H2 + H2O
The HTEL even allows the direct and simultaneous reduction of water and carbon dioxide to synthesis gas within the solid oxide electrolysis cell. This process is called co-electrolysis and is currently also a strong focus of HTEL research.
H2O + CO2 → H2 + CO + O2
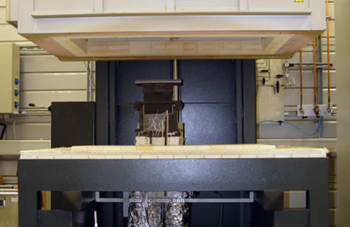
At the CUTEC Research Center, high-temperature steam and co-electrolysis are investigated both at the cell and stack level. Two different test benches are available for this purpose. With the fully automated stack test stand (Fig. left), cell stacks of up to 15 cells and a power of up to 5 kW can be operated in both electrolysis and fuel cell mode. The temperature distribution within the stack, the pressure drop and the individual cell voltages can also be measured in long-term tests.
The second test stand (Fig. right) allows the investigation of single cells with an area of 25 cm² as well as stacks of up to five such cells. The test stand has an innovative sealing concept so that a gas tightness of up to 95 % can be achieved without the use of a glass plummet. This means that the cells can be exchanged without destruction and consequently even reused. Furthermore, the associated potentiostat enables EIS measurements to be carried out, whereby the potential of the cell can be tapped at different points on the cell surface by means of eight additional voltage outputs. With the help of the existing measurement technology, it is possible to detect inhomogeneous potential distribution on the cell surface, to determine kinetic parameters of the electrode reactions and to measure mass transport limitations.